Define Addition Reaction Class 10
The atomic number of carbon is 6. Molecules with carbonhetero double bonds such as carbonyl CO or imine CN groups can be added.
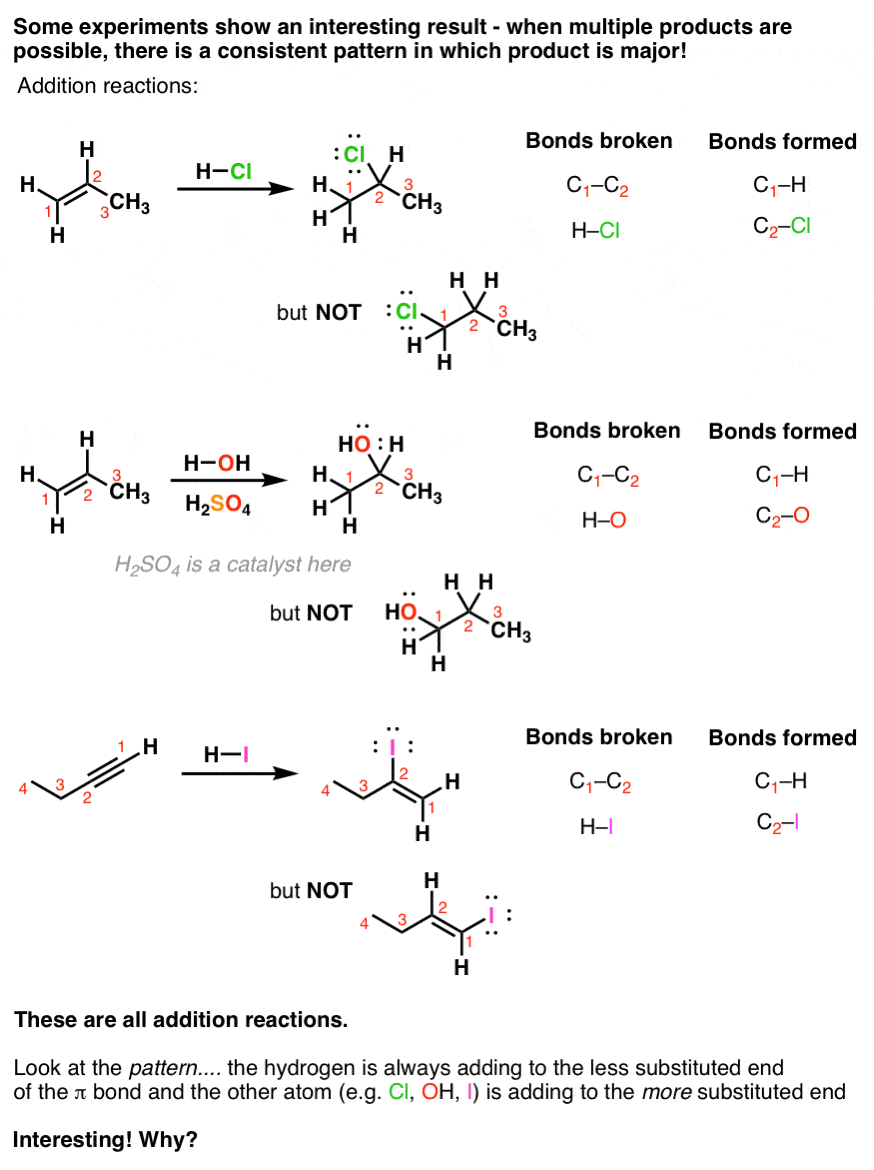
Introduction To Addition Reactions Master Organic Chemistry
Define with examples addition reactions oxidation reactions combustion reaction.

Define addition reaction class 10. CH 2 CH 2 H 2 Nickel catalyst CH 3 CH 3. Saponification definition saponification reaction saponification value are some of the important topics for your CBSE Class 10 Board Exam perspective also. The decomposition of carbonic acid in soft drinks which can be represented by the chemical equation H 2 CO 3 H 2 O CO 2.
The transformation of chemical substance into another chemical substance is known as Chemical Reaction. Addition reactions are limited to chemical compounds that have multiple bonds such as molecules with carboncarbon double bonds alkenes or with triple bonds alkynes and compounds that have rings which are also considered points of unsaturation. In organic chemistry an addition reaction is an organic reaction in which two or more molecules combine to generate a bigger one the adduct.
Disproportionation reaction It is a type of redox reaction where a single reactant is reduced and oxidized. Some common examples of decomposition reactions are provided below. Decomposition reactions happen all around us but we often dont notice them.
Combustion reaction It is a type of redox reaction which occurs between molecular oxygen and compound to form oxygen-containing products. Halogenation is a good example of a replacement reaction. Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings.
Addition reaction any of a class of chemical reactions in which an atom or group of atoms is added to a molecule. The Covalent bond Electron dot structure Physical properties of organic compounds Allotropes of Carbon. Class 10 Chemistry Chemical Reactions and Equations.
You will perform soap making practical as well in chemistry lab of your school in class10. Class 10 Chemistry Chemical Reactions and Equations. Ethene is converted into ethane when heated with the catalyst nickel.
Depending on the reaction kinetics elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the. Imagine that you are given two sets of wooden planks and asked to make something out of them - anything you want. C-C pi bond is formed.
In exothermic reactions the reactants always possess more energy than the products and hence are less stable. The elimination reaction consists of three fundamental events and they are. Formation of larger molecules by addition of more radicals is known as addition reaction.
Its electronic configuration is 2. You get out your glue saw nails and hammer and start building. A reaction involving the exchange of ions between the reactants is termed as Double displacement reaction.
When ethene undergoes addition reaction with chlorine it gives dichloroethane. A reaction in which one reactant undergoes oxidation whereas the other gets reduced during the course of reaction are termed as oxidation-reduction reactions or redox reactions. Consider the following double displacement reaction.
Addition of hydrogen or removal of oxygen from a substance. Substitution reaction is also known as single displacement reaction or single replacement reaction is a chemical reaction during which one functional group is replaced by another functional group in a chemical compound. An addition reaction in organic chemistry is in its simplest terms an organic reaction where two or more molecules combine to form a larger one the adduct.
There is a breakage in the bond of the leaving group. It is also known as an auto-oxidation reaction. Rusting of iron the setting of milk into curd digestion of food respiration etc.
Chemical reaction between Na 2 S and HCl. For this reason the exothermic reactions require very less amount of activation energy to initiate the reaction. What is a chemical reaction Class 10.
Carbon and its Compounds Notes CBSE Class 10 Science Chapter 4. Class 10 chemistry please do Get the answers you need now. Addition reactions are typical of unsaturated organic compounds ie alkenes which contain a carbon-to-carbon double bond and alkynes which have a carbon-to-carbon triple bondand aldehydes and ketones which have a carbon-to-oxygen double bond.

Addition Reaction Definition Example Video Lesson Transcript Study Com

Addition Reaction Definition Examples And Mechanism

Addition Reaction Chemical Reaction Britannica

Addition Reaction Definition Examples And Mechanism

Michael Addition Reaction Details Mechanism Examples Faqs

Markovnikov S Rule Definition Explanation Of Mechanism With Examples
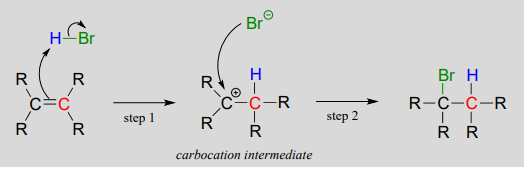
14 2 Electrophilic Addition To Alkenes Chemistry Libretexts
Difference Between Addition And Substitution Reactions Definition Types Characteristics Examples Comparison

Addition Reaction Definition Examples And Mechanism
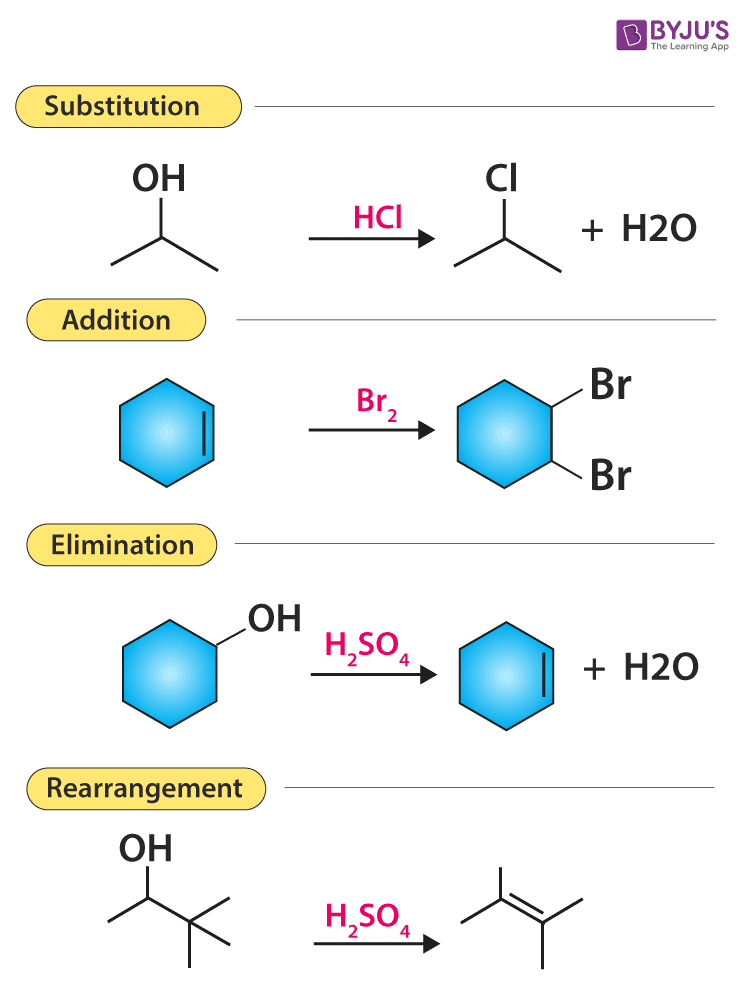
Various Types Of Organic Reactions Polar And Radical Reaction
Difference Between Addition And Substitution Reactions Definition Types Characteristics Examples Comparison
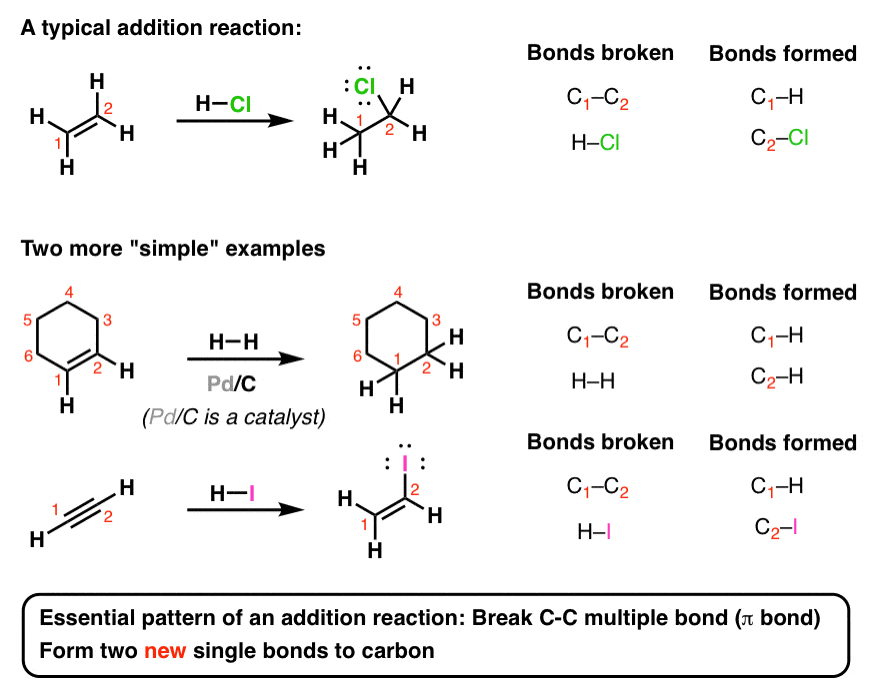
Introduction To Addition Reactions Master Organic Chemistry

Addition Reaction Definition Examples And Mechanism
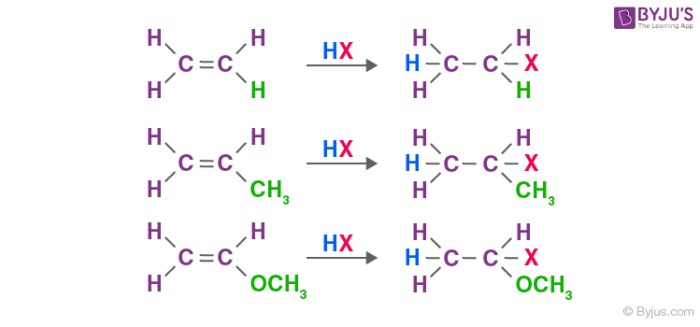
Electrophilic Addition Reactions Of Alkenes Electrophilic Substitution With Examples

Addition Reaction Definition Example Video Lesson Transcript Study Com

Addition Reaction Definition Examples And Mechanism

Addition Reaction Definition Example Video Lesson Transcript Study Com
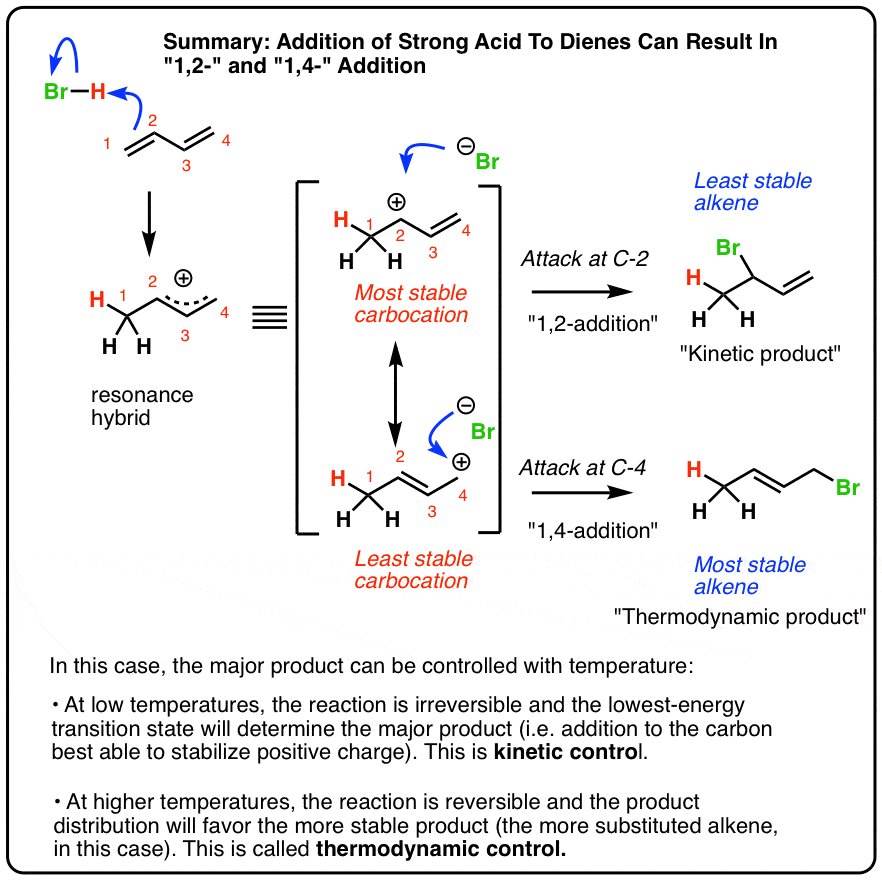
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry
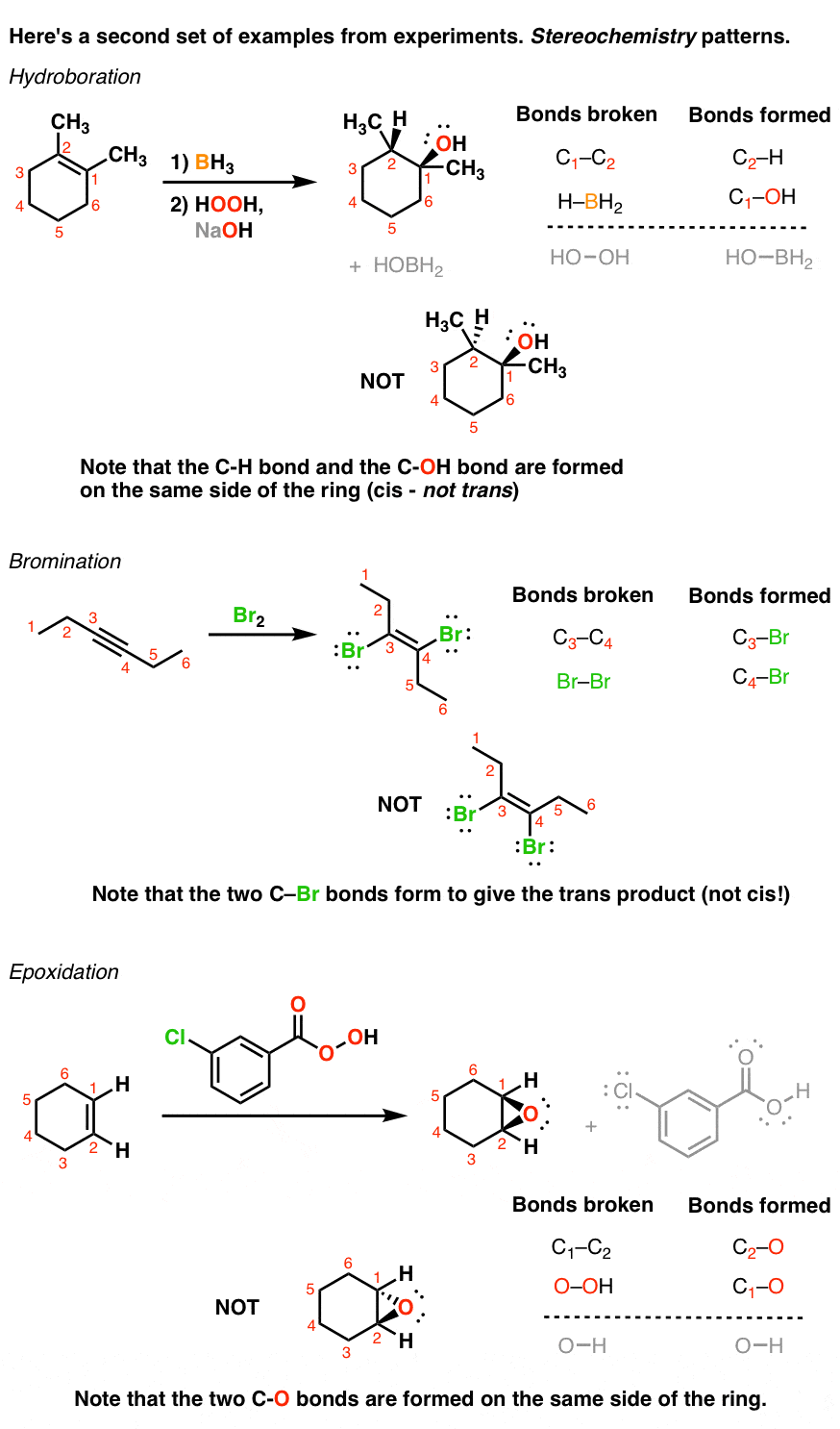
Introduction To Addition Reactions Master Organic Chemistry